Abstract
Background: Recently, direct oral anticoagulants (DOACs) have been investigated as an alternative to low-molecular-weight heparin (LMWH) for the treatment of cancer-associated venous thromboembolism (VTE). Apixaban, edoxaban, and rivaroxaban showed non-inferiority in preventing recurrent VTE compared with the dalteparin. However, it was reported higher risk of bleeding in gastrointestinal (GI) cancers in patients treated with edoxaban and rivaroxaban compared with dalteparin, but not with apixaban. It has been suggested that non-resected GI cancers have higher risk of bleeding than resected ones. The CAP study investigated apixaban as a treatment for cancer associated VTE (1). In this subanalysis, we aimed to investigate risk factors for bleedings and to find out if reducing the dose of apixaban influenced bleedings.
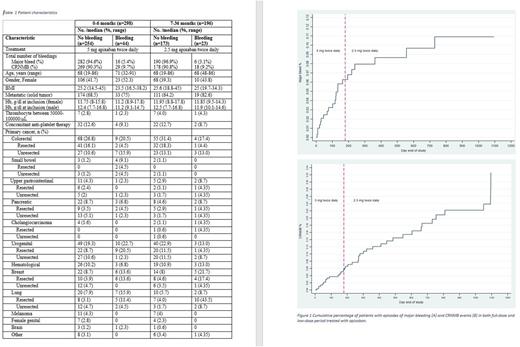
Methods: The CAP study was a prospective, single-arm, interventional study conducted in Norway to assess the efficacy and safety of apixaban as a treatment for cancer-associated VTE. Patients received initially 7 days with 10 mg apixaban twice daily followed by 5 mg twice daily ("full dose") from baseline to 6 months, and 2.5 mg twice daily ("low dose") from 7 to 36 months. Bleedings were categorized as major bleedings or clinically relevant non-major bleedings (CRNMB). The combination of major bleedings and CRNMB is referred to as "clinically relevant bleedings” (CRBs). We investigated the following risk factors for bleeding: age, gender, low BMI, metastasis, unresected tumor, low thrombocyte count, cancer type, low hemoglobin, and concomitant use of anti-platelet therapy. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to assess the association between risk factors and bleedings.
Results: Patient characteristics and bleeding events by type of cancer are shown in table 1. We found that CRBs were associated with high age (above 74 years old, OR 2.0, 95% CI 1.0-4.1), low BMI (below 21.7, OR 2.3, 95% CI 1.1-4.8), and low hemoglobin (below 10.5 for females, OR 2.8, 95% CI 1.1-7.3 and 11.1 for males, OR 3.3, 95% CI 1.3-8.4), during full dose apixaban treatment period. Having GI or urogenital cancer were not associated with CRB compared with other cancers. In general, patients with unresected cancers did not have more bleedings than resected cancers. However, unresected GI cancer had increased risk of bleeding (OR 3.4, 95% CI 1.0-11.6) compared with resected GI cancer. Within the group of urogenital cancer, resected cancers were apparently associated with bleeding (OR 11.1, 95% CI 1.3-94.0), although with a large confidence interval. We did not find any risk factors associated with CRB from 7-36 months except for having breast cancer (OR 3.2, 95% CI 1.0-10.0). The frequency of major bleeding events was higher in full-dose period compared to the low-dose period but the frequency of CRNMB was not influenced by the reduction in apixaban dose (figure 1). There was one fatal bleeding in the full-dose period.
Conclusion: Risk factors for bleeding in treatment with full dose apixaban were high age, low BMI, low hemoglobin. Low dose apixaban apparently reduced major bleeding events, but not CRNMB.
Reference: 1. Larsen TL, Garresori H, Brekke J, Enden T, Frøen H, Jacobsen EM, et al. Low dose apixaban as secondary prophylaxis of venous thromboembolism in cancer patients - 30 months follow-up. J Thromb Haemost. 2022;20(5):1166-81.
Disclosures
Hussaini:Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Decibio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Diaceutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Guidepoint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Tegus: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Larsen:Pfizer: Honoraria; Bristol Myers Squibb: Honoraria. Ghanima:Novartis, Amgen, Sobi, Argenx, UCB, Grifols, Bayer, Hutchmend, Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel and Accommodations, Research Funding. Dahm:Pfizer, Novartis, BMS: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal